Advancement towards more precise and personalized cardiac diagnostics is reinforced by the recent approval of Echo IQ by the FDA through the process 510(k) for his artificial intelligence dedicated to the analysis of heart diseases. The move marks a significant step in the integration of innovative technologies within medical practices, promising to improve patient care by facilitating risk identification and optimizing clinical workflows. Echo IQ is now positioned as a key player in the field of heart health thanks to this official recognition.
|
IN BRIEF
|
Recently, Echo IQ reached a major milestone with obtaining approval 510(k) of the FDA for its revolutionary artificial intelligence (AI) dedicated to the detection of heart disease. This approval marks a significant turning point in the integration of advanced technologies into medical practices, thus enabling more precise and rapid diagnosis of cardiac pathologies.
A step forward for heart disease detection
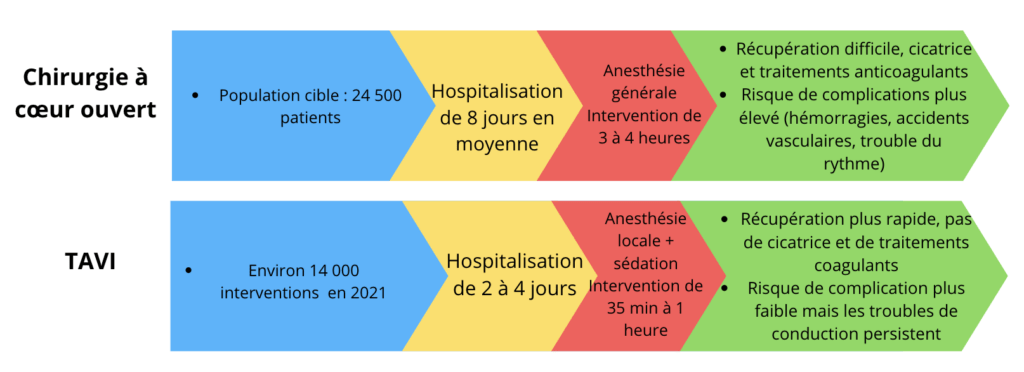
The AI developed by Echo IQ was designed to automatically identify patients at risk of structural heart disease. With this innovation, practitioners can now benefit from a sophisticated tool that significantly improves workflow, making diagnosis more efficient. The integration of AI in the field of cardiac ultrasound strengthens the first line of defense against cardiovascular conditions, a necessity in the face of the growing increase in these diseases.
Technology at the forefront of innovation
Technological advances in the field of medical imaging, particularly ultrasound machines, have opened the way to more complete diagnostics. The 510(k) approval recognizes not only the effectiveness of the device, but also the growing need for artificial intelligence tools like Echo IQ, which meet the challenges of more personalized and preventative medicine.
Indispensable support for the development of medical applications
The formalization and filing of 510(k) files with the FDA is a complex process, requiring expertise and precise advice. Echo IQ successfully navigated this path to obtain the necessary authorizations, demonstrating its ability to meet the rigorous standards imposed by health authorities. To learn more about this process, it is possible to consult specialized sources such as Isocele Medial.
The implications of this approval for the medical sector
This milestone in Echo IQ’s journey highlights significant implications for the medical sector in terms of early diagnosis and treatment management. AI not only makes it possible to optimize diagnoses, but also to offer more appropriate therapeutic interventions thanks to better identification of risks. The impact of the use of AI in medical diagnosis on practitioners and patients cannot be underestimated, as demonstrated by the recent analysis on artificial intelligence medical diagnostics.
Promising future prospects
With innovative projects within the sector, such as AUDIOCARE ILAB 2024 which incorporates AI analysis of sound for mood disorders, it is evident that AI is revolutionizing the healthcare landscape. The presence of advanced artificial intelligence tools like Echo IQ could become the norm, strengthening our understanding and management of heart disease. For a more detailed look at new advances, check out articles published on platforms such as Zonebourse.
🇬🇧🧑🏼⚕️Une nouvelle technologie d'IA développée par Caristo Diagnostics peut détecter à l'avance les risques cachés de crise cardiaque en analysant les tomodensitogrammes pour détecter l'inflammation coronarienne.
— Edith Brou Bleu (@edithbrou) August 6, 2024
📍Un projet pilote, soutenu par le NHS England, est en cours dans… pic.twitter.com/KYGbEsQCUw
Echo IQ recently got the510(k) approval of the FDA for its major innovation: a artificial intelligence dedicated to heart disease. This validation is an important milestone in the field of health, testifying to the effectiveness and safety of this tool in the medical diagnosis process. With this approval, Echo IQ establishes itself as a key player in themedical innovation, by combining advanced technology and clinical expertise.
By integrating machine learning algorithms to its solutions, Echo IQ revolutionizes the diagnosis of heart diseases by allowing early identification patients at risk. The algorithm was designed to quickly and accurately process complex data, significantly improving data processing practices. diagnosis. Furthermore, a process of workflow optimized is proposed, thus minimizing the time spent by healthcare professionals analyzing the results.
This technological advance is all the more relevant in a global context where heart disease represents one of the leading causes of mortality. Echo IQ’s ability to combine analytical power and ease of use could ultimately help reduce diagnostic times and improve patient monitoring.
510(k) approval also increases healthcare professionals’ confidence in artificial intelligence tools. As part of a holistic strategy, Echo IQ aims to integrate this innovative technology into the daily life of healthcare establishments, thus transforming the way in which heart disease are diagnosed and treated.