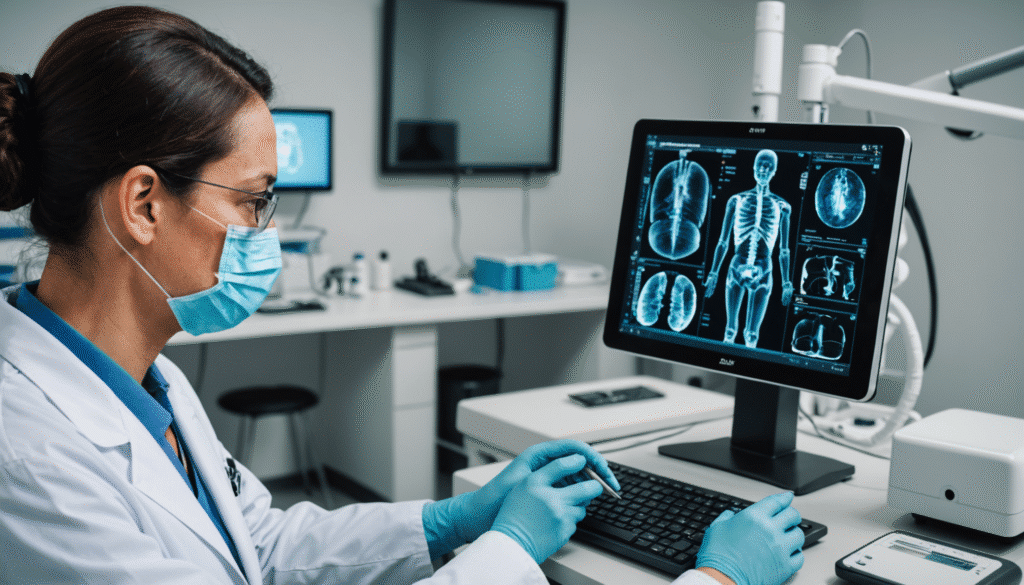
The recent European regulations concerning medical technologies are sparking heated debates within the sector. According to many companies, these strict rules significantly limit the options available for patients, thus hindering innovation and access to essential medical devices. Small businesses, in particular, find themselves under increased pressure as the regulatory requirements seem more suited to large multinationals, leaving little room for the creativity and flexibility needed to meet the specific needs of users. At the heart of these concerns, the desire to improve the health of Europeans sometimes contradicts the goal of broader choice and greater accessibility.
The European regulations regarding medical technologies are facing strong criticism from industry companies. They believe that these rules do not promote innovation and restrict patients’ access to new devices. According to several testimonies, complicated compliance procedures particularly burden small businesses, limiting their ability to market health-benefiting products. Industry players advocate for a revision of the standards to facilitate the market entry of innovative solutions, proposing a more pragmatic approach that meets the needs of patients.

the European regulations regarding medical technologies
Companies in MedTech are expressing growing concerns over the new regulations put in place by the European Union. They believe that these regulations complicate access to medical technologies for patients. Many stakeholders in the industry lament that the complexity of compliance requirements results in significant delays in bringing devices to market. The market is thus limited, directly affecting the availability of innovative treatment options for patients.
impact on innovation and competitiveness
With less freedom to innovate, small and medium-sized enterprises feel particularly affected. These start-ups, which play a key role in the emergence of new medical technologies, must now navigate a complex regulatory environment that hinders their ability to grow. Their limited resources make it difficult to meet the requirements, sometimes forcing them to abandon promising projects.
reactions and perspectives of industry stakeholders
Comments from companies have highlighted a general desire for a revision of the current regulations. Many advocate for a more sensitive approach that would encourage a balance between safety and inaccessibility of care. For example, stakeholders like ACI Medical assert that financial requirements prevent them from providing vital devices for European patients.